Introduction
Blockchain in Pharmaceuticals Statistics: Most people, including me, imagine new drugs or advanced treatments when we say the future of medicine, but behind it, there’s another term making changes, blockchain. I hope you have already heard of that term. It is already changing finance and supply chains, but it is now entering healthcare too.
The number already proves it, that’s why I’d like to explain everything about the blockchain in pharmaceutical statistics. What surprises me is, counterfeit drugs, weak supply chains, and clinical trial fraud have troubled pharma for years. These statistics around its adoption tell a clear story; let’s break down everything.
Editor’s Choice
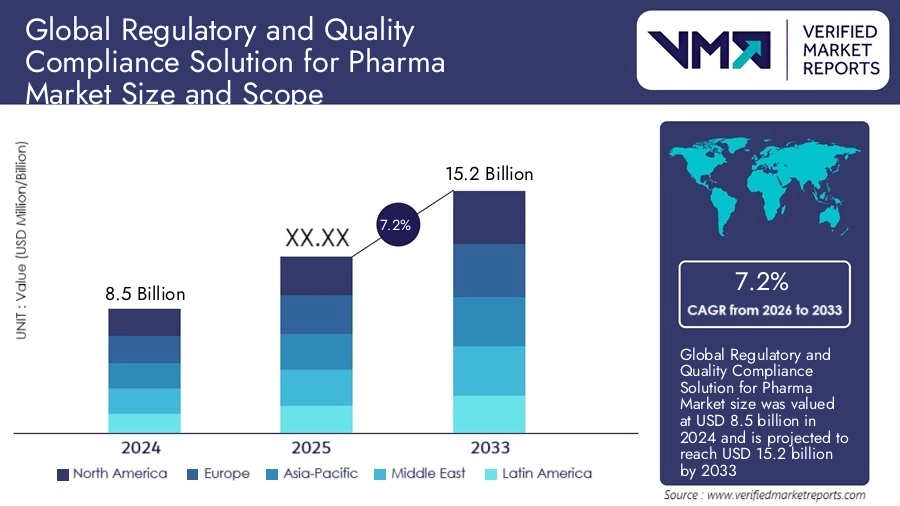
- The global market for blockchain in pharmaceuticals was valued at USD1.3 billion in 2023 and is projected to hit USD6.5 billion by 2028.
- Nearly 20% of drugs worldwide are counterfeit, causing annual losses of over USD200 billion, and blockchain is tracking this problem head-on.
- Around 55% of pharma companies are piloting or already using blockchain for drug traceability and supply chain security.
- Blockchain can reduce drug recall times by up to 70%, making patient safety stronger than before.
- Clinical trials. Account for 40% of pharma R&D spending, and blockchain adoption is helping cut fraud and data manipulation cases significantly.
- By 2030, smart contracts in blockchain could save the pharma industry nearly USD150 billion in compliance and operational costs.
- Over 60% smart contracts in blockchain could save the pharma industry nearly USD150 billion in compliance and operational costs.
- Over 60% of healthcare executives believe blockchain will become a standard tool in securing sensitive drug and patient data.
- In Asia-Pacific, adoption is rising fastest, with blockchain in pharma expected to grow at a compound annual growth rate (CAGR) of 75% by 2030.
- Blockchain pilots by companies like Pfizer, Novartis, and Merck show up to 90% improvement in transparency across supply chains.
- By 2025, nearly 30% of clinical trial data could be stored on blockchain, reducing research duplication and increasing trust.
| Area | Metrics | What it Means |
| Global Market Size | $1.3B in 2023 to $6.5B by 2028 |
Rapid growth shows blockchain is becoming mainstream in pharma |
|
Counterfeit Drugs |
20% of global supply, $200B losses | Blockchain ensures authenticity and safety |
| Supply Chain | 55% of companies are adopting blockchain |
Stronger traceability and security |
|
Drug Recalls |
70% faster with blockchain | Saves lives and cuts financial losses |
| Clinical Trials | 40% of R&D spend + blockchain storage by 2025 (30% data) |
Increases transparency, reduces fraud |
|
Cost Savings |
$150B by 2030 from smart contracts | Efficiency in compliance and operations |
| Regional Growth | APAC CAGR 75% by 2030 |
Fastest-growing adoption region |
|
Pharma Leaders |
Pfizer, Novartis, Merck pilots | Up to 90% more transparency |
| Data Security | 60% of executives see blockchain as standard |
Stronger protection of patient and drug data |
What is Blockchain in Pharmaceuticals?
 (Source: tudip.com)
(Source: tudip.com)
- The roots are simple. 2008 gave us the Bitcoin paper, then enterprises started building permissioned chains.
- From 2016 to 2017, pharma pilots popped up with track and trace and temperature sensing.
- 2019 saw EU FMD go live on end-user verification and U.S. DSCA saleable returns verification.
- 2020 was the FDA pilot year everyone cites; one consortium showed it could trace prescription products across multiple parties within seconds.
- 2022 to 2024 brought country platforms like UAE Tatmeen online, and U.S. trading partners shifted to EPCIS data exchange ahead of the DSCSA interoperability data.
- 2025 is now the stabilization period end target in the U.S, with companies finishing EPCIS connections, verification routing, and exception handling to hit full compliance.
| Milestone | What Happened |
| 2016 to 1017 | Early pharma pilots with IoT plus blockchain |
| 2019 | EU FMD live, U.S. DSCSA saleable returns |
| 2020 | FDA blockchain pilot proved fast traceability |
| 2022 | National platforms expand |
| 2025 | U.S. DSCSA stabilization ends |
Why does it matter?
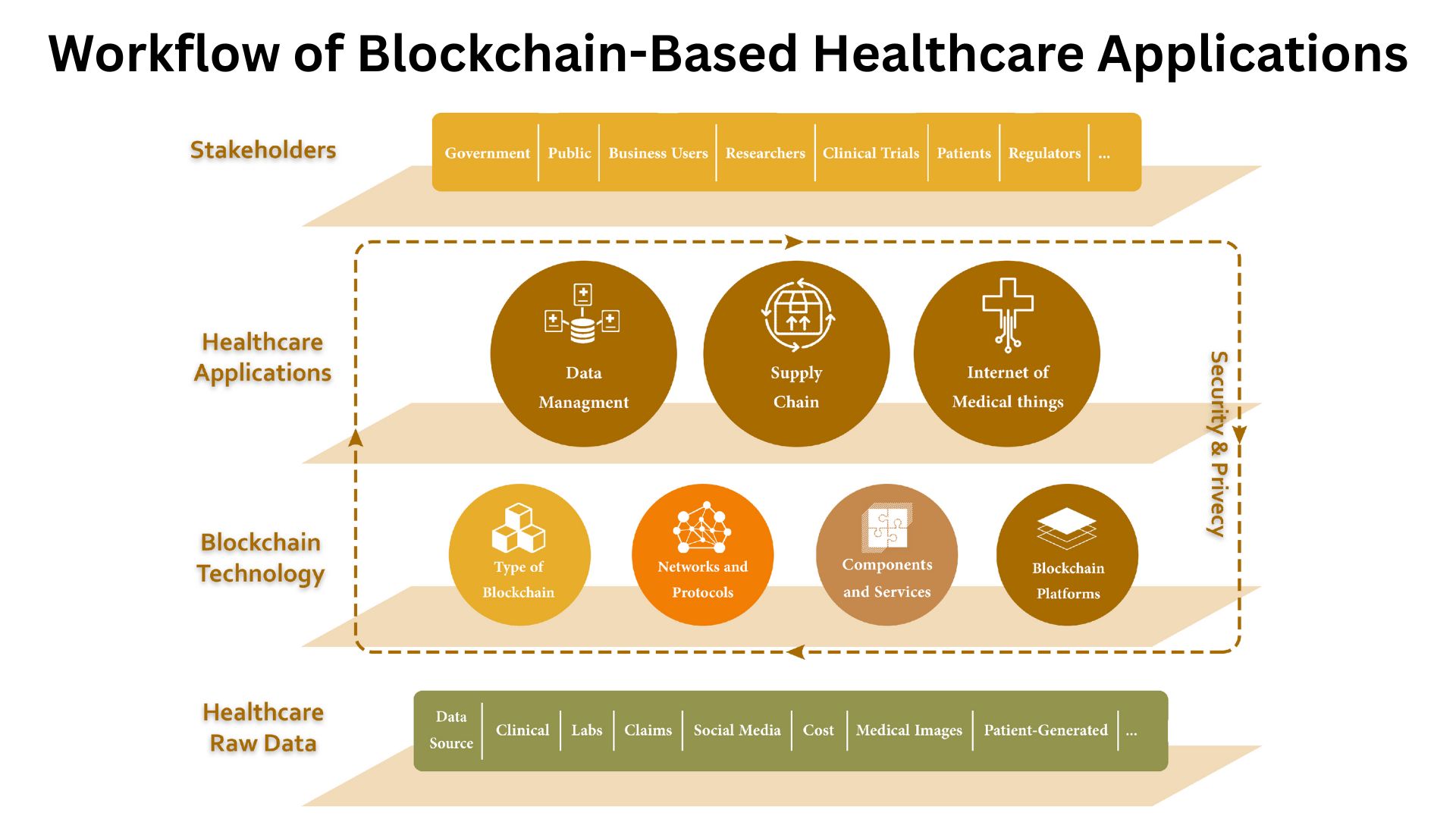 (Source: mdpi.com)
(Source: mdpi.com)
- Counterfeits and substandard meds are not abstract. The global evidence is ugly and persistent, with 10% prevalence in low and middle-income countries.
- Verification at the edge is now feasible; Europe’s end-user verification model is expected to hit more than 10 billion pack verifications in 2025.
- Regulated returns are a giant vector; the U.S. alone has nearly 7 billion dollars of saleable returns every year, and every unit needs verification with the manufacturer before re-entry.
- Logistics and serialization networks link 290,000 stakeholders who exchange EPCIS and verifications.
| Risk or driver | Metrics |
| Counterfeits | 1 in 10 substandard or falsified in LMICs |
| Verification volume | 108 pack verifications expected in 2025 |
| Returns in the U.S. | Nearly 7B dollars in returns annually |
| Network density | 291k entities on a leading network |
Regulation and Deadlines
 (Source: verifiedmarketreports.com)
(Source: verifiedmarketreports.com)
- S. DSCSA is the anchor. Unit-level traceability and interoperable EPCIS exchange are required.
- FDA announced a stabilization period through November 27, 2025, for enhanced security provisions.
- EU FMD keeps scanning at dispensing, the model is repository-based and forces safety feature checks at pharmacies.
- Gulf region is moving fast, the UAE Tatmeen platform is live and scaling. The 2023 is tracked 280 million transactions and 67 million parcels.
- Global serialization continues in waves, local specs vary, but GS1 identifiers, 2D DataMatrix, and EPCIS are the common language that any serious blockchain deployment must speak.
| Region | Key Dates |
| United States | Stabilization to Nov 27, 2025 |
| European Union | Billions of scans each year |
| United Arab Emirates | 280M transactions in 2023 |
| Global Baseline | Common rails across markets |
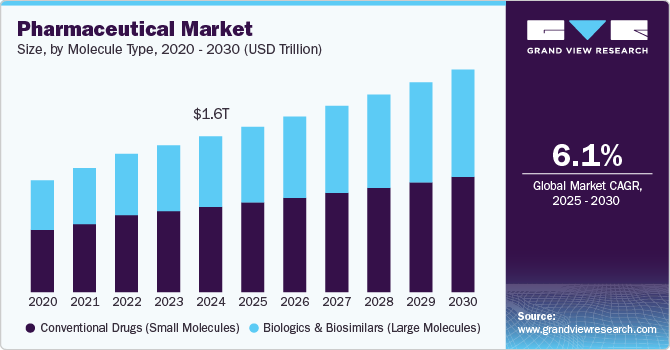
Market Size and Spend
 (Source: grandviewresearch.com)
(Source: grandviewresearch.com)
- Market sizing varies depending on whether you slice “blockchain in healthcare” or the narrower pharma supply chain use.
- Several trackers peg healthcare blockchain in the low billions mid-decade with double-digit CAGR.
- Use-case budgets tend to be program line items under serialization, chargebacks, or quality, not a standalone blockchain budget.
| Tracker | Scope | 2024 to 2028 Metrics |
| Multiple industry trackers | Healthcare blockchain | Low billion-size, strong CAGR |
| Enterprise programs | Serialization plus returns | Spend blended into DSCSA and FMD budgets |
| Vendor signals | Networks and hubs | 1,800 to 1,500+ customers on large networks |
Adoption Signals
 (Source: ispe.org)
(Source: ispe.org)
- EPCIS readiness improved materially in the 2024 HDA EPCIS readiness survey. U.S. trading partners showed rising purchase order line accuracy and fewer data issues as companies move to production EPCIS.
- Verification routing matured, the MediLedger verification router service is the industry way for wholesalers to ask manufacturers, “Is this serial valid?” at scale.
- Digital networks hit scale, one network reports more than 291,000 authenticated partners.
| Indicator | 2024 to 2025 status |
| EPCIS data quality | Improving accuracy and exception rates |
| Verification requests | Routed to mfrs at scale |
| Network participants | Serialization and supply chain nodes have about 291k connected entities |
Performance Outcomes from Pilots and Live Runs
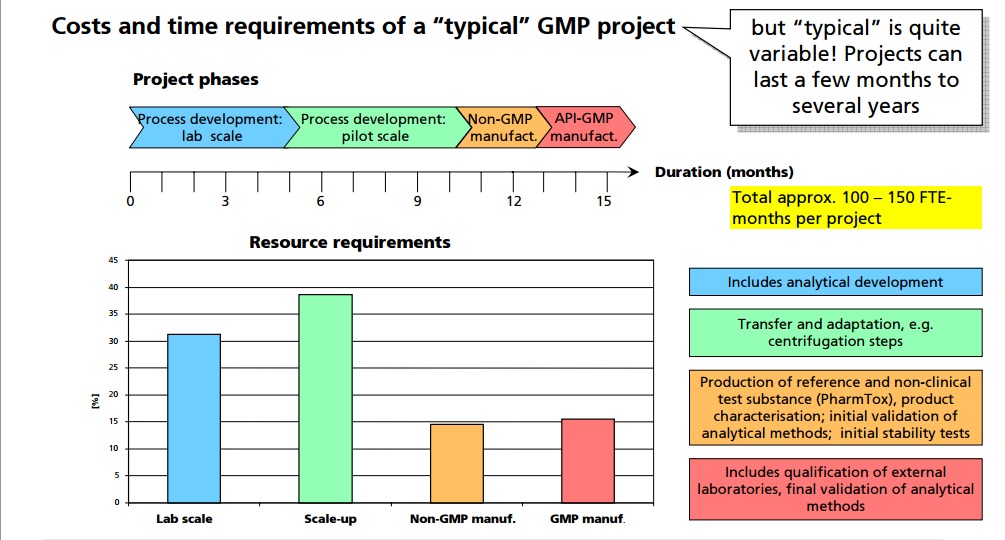 (Source: wordpress.com)
(Source: wordpress.com)
- FDA pilot, 2020, a team with IBM, Merck, KPMG, and Walmart demonstrated an interoperable trace that resolved provenance in seconds across multiple parties.
- Salesable returns verification, because the U.S. processes nearly 7B dollars in returns annually, verification throughout is a day-to-day test.
- UAE Tatmeen 2023 matrics, 280M transactions, 67M parcels, 7,300 products, 546 manufacturers, 601 distributors, 4,613 dispensing facilities, 5,000 active users.
| Program | Measured Outcomes |
| FDA blockchain pilot | End-to-end trace latency is about seconds, not minutes |
| U.S. returns challenge | Verification volume driver, nearly 7B dollars annually |
| SAP ICH live | Production returns verification, live since 2019 |
| Tatmeen UAE | Nationwide transactions in 2023, 280M transactions, 67M parcels |
Clinical Trials, ePI, and Pharmacovigilance
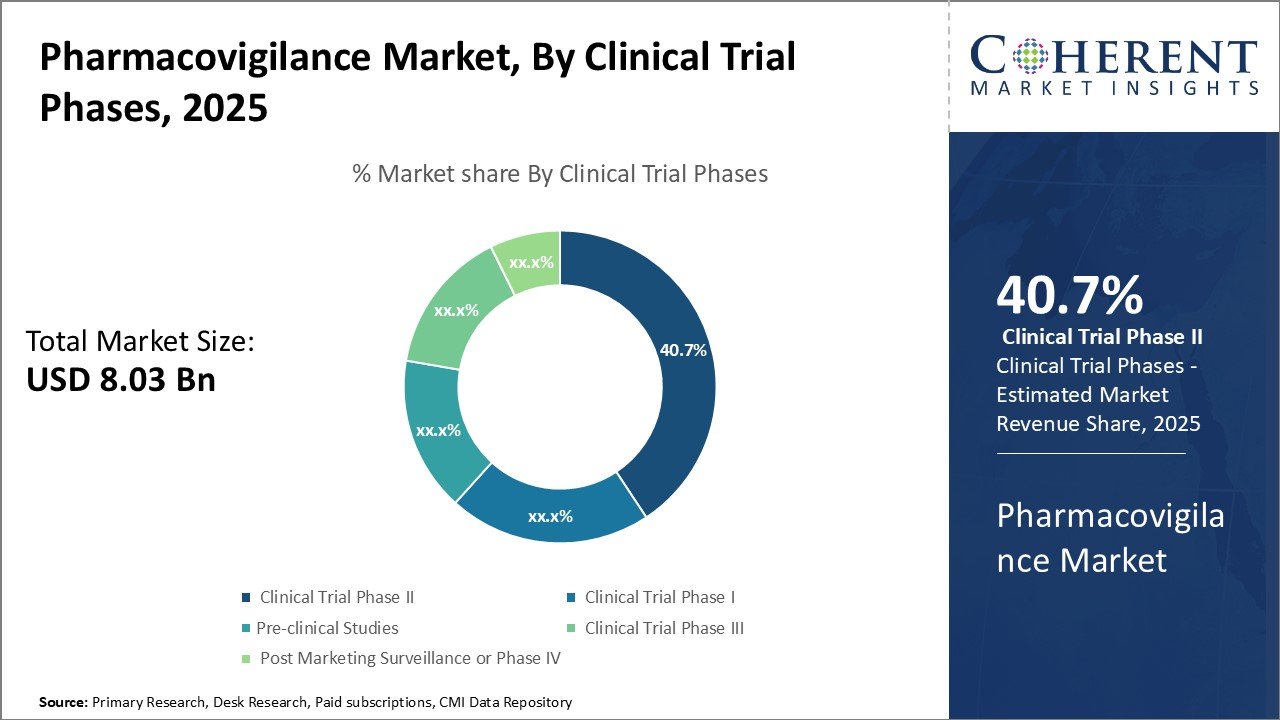 (Source: coherentmarketinsights.com)
(Source: coherentmarketinsights.com)
- Consent and protocol data are obvious blockchain candidates, but pharma is cautious.
- The PharmaLedget public-private project built ePI and other digital health building and ran multi-company pilots that demonstrated feasibility rather than raw transaction scale.
- Today is still stronger on supply chain than on blinded data lakes. Expect growth in ePI distribution tied to serialization codes, so patients scan a 2D code and retrieve the current label in their language with a blockchain-anchored audit.
| Area | What is live vs pilot | Stat or direction |
| eConsent, protocol | Mostly pilots with big pharma | Capability proven, scale pending |
| Electronic Product Information | Pilots and early rollouts | Anchored to GS1 codes and scan data |
| Safety reporting | Identity and integrity benefits | Pharma adopting cautiously |
Cold Chain and IoT Telemetry
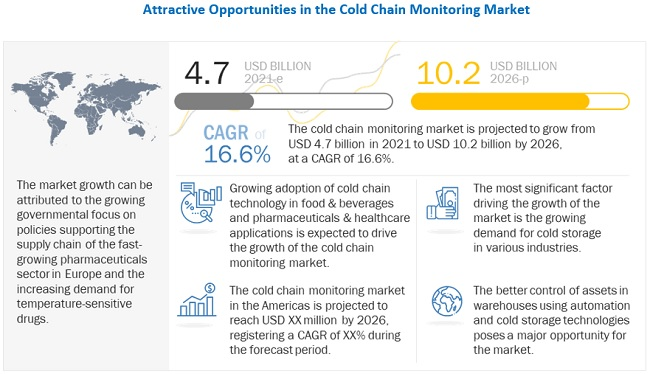 (Source: marketsandmarkets.com)
(Source: marketsandmarkets.com)
- Modum style pilots disposable sensors with a blockchain proof so a receiver can see not just a PDF but a tamper-evident hash anchored to a ledger.
- Vaccine handling studies repeatedly show double-digit excursion rates in weak lanes. When a sensor proves a lane stayed in range, you safely avoid discarding lots that are actually fine, which cuts wastage and rework effort.
| Use Case | What Changes | Number or Takeaway |
| Temperature proof | Replaces paper loggers | Immutable evidence on receipt |
| Lane analytics | Select the best carriers and lanes | Excursion rate reductions drive savings |
| Audit response | Seconds to verify | One hash check instead of doc chase |
Pricing, Contracts, and Chargebacks
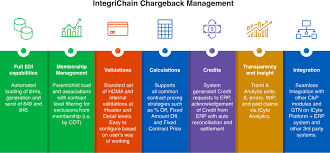 (Source: integrichain.com)
(Source: integrichain.com)
- Chargeback disputes are a daily grind. Industry sources report >100,000 disputes per day in the U.S. due to misaligned pricing and eligibility.
- Smart contracts that sync eligibility, contract terms, and identifiers are cutting that noise.
- Production solutions on MediLedger target error elimination in categories like retroactive price updates, roster misalignments, and product eligibility.
- Dispute and revenue leakage reduction is the KPI teams report internally, not just “we use blockchain.”
| Metric | Baseline pain | Target outcome |
| Disputes per day | 100,000 industry-wide | large reduction through shared rules |
| Error categories | price, eligibility, identifiers | smart contract validation at source |
| Financial impact | leakage and rework costs | fewer credits and faster settlements |
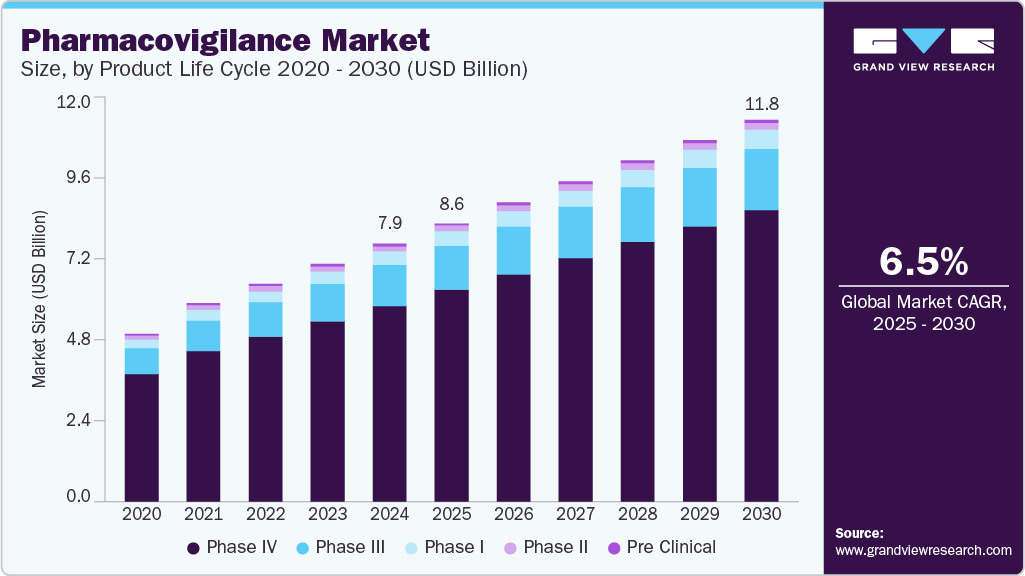
Regional Rollouts that Prove Scale
 (Source: grandviewresearch.com)
(Source: grandviewresearch.com)
- United States. The stabilization policy gives the market until November 27, 2025, to mature EPCIS flows. Expect a step-function improvement in exception handling in the next 12 months.
- European Union. End-user verification keeps producing massive scan volumes and alerts. Repository events can be notarized externally where needed as companies seek cross-border audit portability.
- United Arab Emirates. Tatmeen is an example of a national control tower for medicines using GS1 standards with ledger components. We already saw the 2023 metrics in the millions.
- Other regions. GCC neighbors, parts of Asia, and Latin America are either drafting or phasing in track-and-trace, usually GS1 aligned. Many will skip straight to EPCIS 1.2 or EPCIS 2.0.
| Country or bloc | State of play | Number |
| U.S. | DSCSA interoperability stabilization | through Nov 27, 2025 |
| EU | repository checks at dispense | billions of scans per year |
| UAE | national platform at scale | 280M transactions in 2023 |
| Global | GS1 and EPCIS adoption | 2.0 specs gaining mindshare |
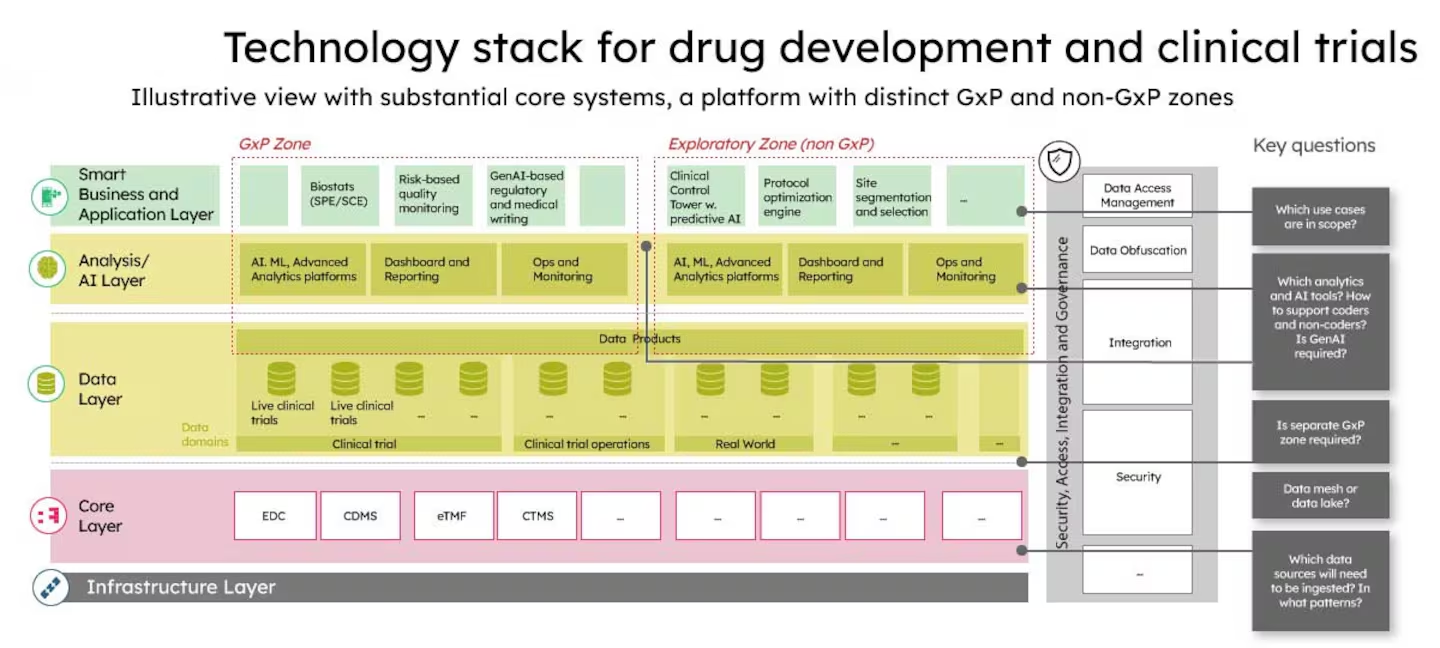
Tech Stack and Standards
 (Source: insights.citeline.com)
(Source: insights.citeline.com)
- GS1 standards are mandatory table stakes. GTINs, serials, lot, expiry, plus EPCIS event data are the payload. Blockchain entries often store hashes or proofs, not raw PHI or trade secrets.
- Verification Router Service connects wholesalers to manufacturers for real-time serial checks. Multiple vendors interoperate here, and it is one of the busiest flows in U.S. drug logistics.
- Enterprise hubs run in parallel. SAP’s Information Collaboration Hub went live for returns verification in 2019, and partnered to reach manufacturers and wholesalers already on blockchain-backed verification.
- Network scale supports latency and resilience. When a network reports 291,000 authenticated partners and 1,800+ customers, that signals critical mass for exchange volumes and redundancy.
| Component | Role | Stat or fact |
| GS1 plus EPCIS | the data language | EPCIS 1.2 today, 2.0 rising |
| VRS | real-time serial checks | standard flow for returns and suspect |
| SAP ICH | hub for returns verification | live since 2019 |
| Digital network | connectivity at scale | 291K partners, 1,800+ customers |
What Adopters Actually Measure
 (Source: ispe.org)
(Source: ispe.org)
- Trace times from the dispenser back to the manufacturer. Pilots reported seconds. Production systems track the 95th percentile latency for EPCIS queries and verification round-trips.
- Data quality on EPCIS feeds. Companies track % of PO-line matches, serial status mismatches per million, and exception backlog days. HDA shows these metrics improved in 2024.
- Dispute rates in chargebacks. Before and after numbers on repeat error codes, average days to resolve, and credit memo cycle time.
Regulatory lead time. Days from alert to quarantine, plus recall notification time to all affected sites.
| KPI | Good sign | What a strong number looks like |
| Trace latency | seconds not minutes | p95 under 5 to 10 seconds |
| EPCIS data quality | fewer exceptions per million | continuous downward trend |
| Chargeback disputes | fewer repeats of top 5 codes | double-digit % drop |
| Recall notice time | faster and broader reach | minutes to all affected nodes |
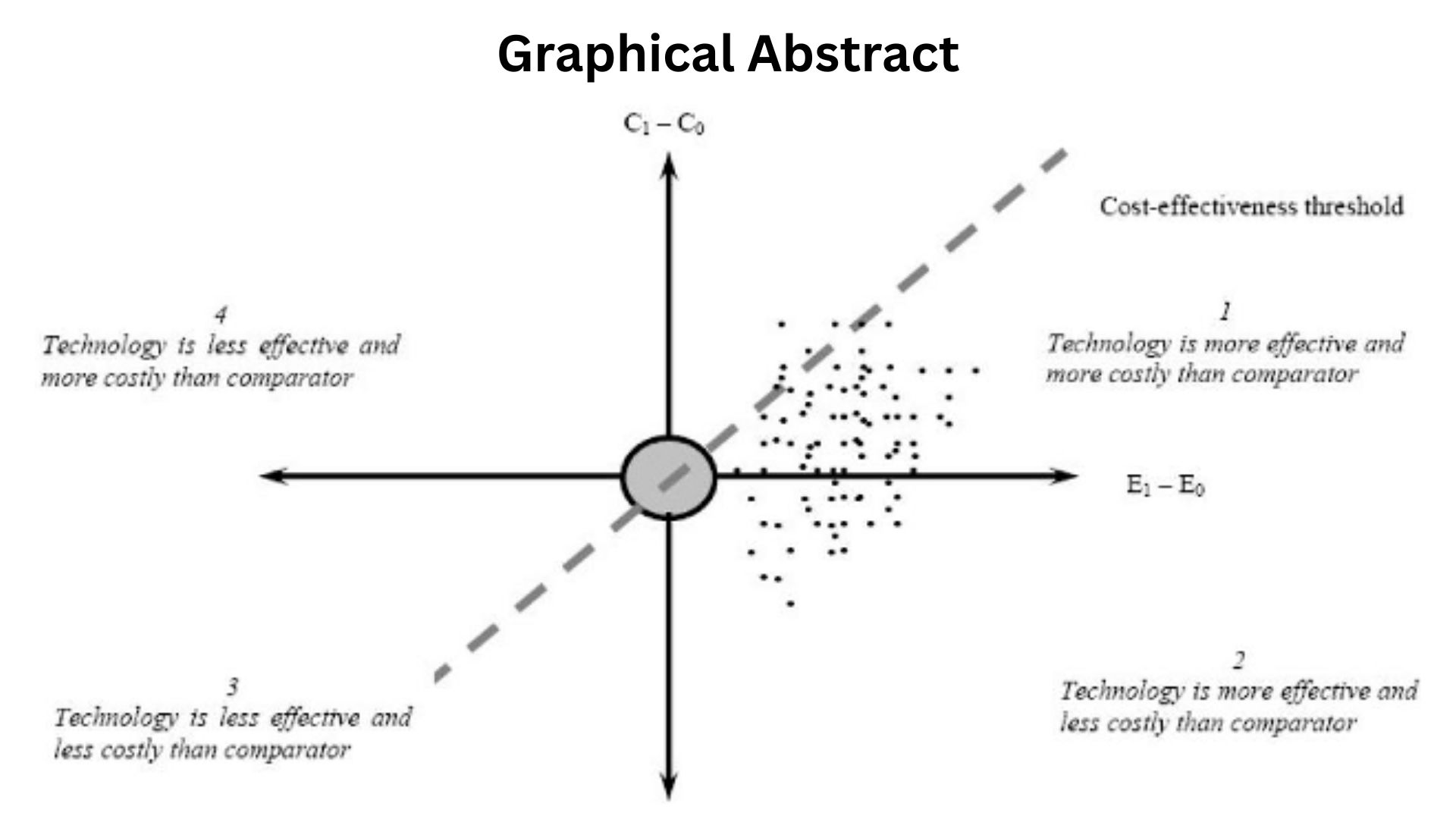
Economics, in Plain Math
 (Source: mdpi.com)
(Source: mdpi.com)
- Counterfeit risk avoided. If a market faces even a low single-digit counterfeit infiltration risk, notarized verification at dispense protects revenue and avoids recall blowback. You measure avoided loss, not just IT cost.
- Returns re-entry. With nearly 7B dollars of saleable returns in play, every percentage point of faster verification that reduces hold time or manual exception labor has a direct working capital effect.
- Chargeback leakage. When 100,000 daily disputes are cut sharply, the P and L signal is visible in fewer credit memos and lower write-offs.
| Cost center | Why blockchain helps | Number anchor |
| Counterfeits | immutability plus verification | 1 in 10 substandard or falsified in LMICs |
| Returns | proof tied to serials and events | nearly 7B dollars annually in U.S. |
| Chargebacks | shared eligibility and pricing truth | 100,000 disputes daily baseline |
Limits, Risks, and What People Get Wrong
 (Reference: mdpi.com)
(Reference: mdpi.com)
- Blockchain is not the product database. It’s a verification layer. Raw EPCIS events live off-chain, and you anchor proofs or route verification requests. Thinking it’s a giant shared table is how projects fail.
- Privacy matters. Commercial price lists, 340B eligibility details, and patient identifiers do not belong on the chain. Use selective disclosure and strict off-chain storage.
- Throughput and cost. For this vertical, permissioned networks with routing services are the norm because you need predictable latency and finite participants.
- Vendor lock-in risk. Mitigate by committing to GS1 and EPCIS standards. You should be able to switch a verification or EPCIS gateway without a full replatform.
| Risk | Practical response | Metric to watch |
| Data spill | off-chain data, on-chain proofs | zero confidential fields on chain |
| Latency spikes | private networks and caching | p95 response time in seconds |
| Lock-in | standards first, vendor second | EPCIS conformance results |
| Cost overrun | scope trace plus 1 or 2 adjacencies | ROI by dispute and rework cuts |
What to Expect in the Next 12 Months?
 (Source: dcatvci.org)
(Source: dcatvci.org)
- S. DSCSA stabilization ends Nov 27, 2025. Expect production EPCIS volume to climb, plus fewer manual exceptions and cleaner master data.
- UAE Tatmeen will keep adding partners and flows. National numbers already show hundreds of millions of transactions per year.
- EU will keep high verification volumes and more analytics around alert rates and decommission events.
- Vendor ecosystem. Verification routing, SAP ICH, and large multi-enterprise networks will expand their SKU coverage and speed.
| Milestone | Date | What to watch |
| U.S. DSCSA stabilization end | Nov 27, 2025 | exception rate curve, EPCIS volume |
| UAE Tatmeen scale | ongoing | transaction counts and partner growth |
| EU repository analytics | ongoing | scan alerts and false positive rate |
| Network expansion | 2025 | partner count and latency stats |
Implementation Checklist
 (Source: researchgate.net)
(Source: researchgate.net)
- Map your EPCIS flows. Count every commission, pack, ship, receive, and decommission event path per product family and lane. The count tells you the throughput planning.
- Target p95 under 10 seconds for verification and trace queries. If your architecture cannot meet that, fix routing, caching, or master data.
- Measure disputes by the top 5 error codes before and after go-live. You want a double-digit % reduction in three months; otherwise, adjust validation rules.
- Onboard key partners first. Your first 20 partners probably cover 80 % of your volume. Sequence by volume and data quality, not politics.
| Step | Numeric target | Why |
| EPCIS mapping | 100% flows are known | capacity and testing |
| Verification SLA | p95 under 10s | user experience and throughput |
| Dispute reduction | double-digit % drop | ROI you can show |
| Partner onboarding | top 80 % volume first | fast impact on exceptions |
Conclusion
So, overall, the pharmaceutical world is changing, it’s clear that blockchain is no longer is future. It’s becoming a backbone for trust, safety, and efficiency in the way medicines are made, tested, and delivered. From fighting counterfeit drugs to speeding up recalls and making clinical trials more transparent, blockchain is slowly reshaping the industry step by step.
This is still in its early stages, but the signs are powerful. As more pharma companies, regulators, and healthcare providers lean into this technology, patients will be the real winners, getting safer medicines, faster treatments, and greater trust in the system. Blockchain in pharmaceuticals isn’t just about data on a ledger; it’s about building a future where healthcare is more reliable, secure, and human-centered. I hope you guys love this article. If you guys have any questions, kindly let me know in the comments section. Thanks.


